Category
Subcategory
Manufacturer

ultraWAVE 3 SRC Microwave Digestion System 3rd generation
Single Reaction Chamber Technology The new ultraWAVE 3 is the latest generation of SRC technolog...

ETHOS™ UP High Performance Microwave Digestion System
The ETHOS UP fully embodies Milestone’s philosophy towards, and knowledge of, microwave sample pr...

ETHOS™ X Environmental Microwave Extraction System
Streamlining Environmental Lab Workflows with the Milestone ETHOS X Your feedback has driven the...

SER 158 Series Automatic Solvent Extractor
Fast and accurate solid-liquid extraction The SER 158 series of automatic solvent extractors in ...

Horizontal Sieve Shaker AS 400 Control
The RETSCH AS 400 control is used for sieving dry goods with test sieves up to 400 mm in diameter...

ELEMENTRAC CN-r Carbon / Nitrogen / Protein Analyzer
The ELEMENTRAC CN-r is the perfect solution for high-throughput laboratories needing fast and rel...

HU 6 Hydrolysis Unit
Convenient, reliable and safe hydrolysis unit, for the sample preparation prior to extraction for...

SER 148 Series Solvent Extractors
Safe and convenient semi-automated extraction system suitable for fat extraction and sample prepa...

FIWE Fiber Analyzers
Crude Fiber, Neutral & Acid Detergent Fiber FIWE 3 and FIWE 6 are fiber analyzers suitable for r...

Ultra Centrifugal Mill ZM 300
The latest model ZM 300 comprises the essence of German engineering expertise combined with high-...

synthWAVE Single Reaction Chamber microwave Synthesis System
The new Milestone synthWAVE is designed for safe,reliable and reproducible scale-up of microwavee...

Nanotrac Wave II Q Nanoparticle Size Analyzer
Using Reference Beating, an enhancement to traditional DLS, the Nanotrac Wave II Q delivers an am...

Two-Station Dosing and Filling Machine FDF
The two-station dosing and filling unit is suitable for the drop-free filling of all types of dif...

Dosing and Filling Machine FDS
Dosing Machine FDS is suitable for filling precise quantities of ointments, lotions, emulsions an...

Mixer Mill MM 500 Vario
Processing 2 to 50 samples in one batch The new Mixer Mill MM 500 vario is a versatile bench-top...

Mixer Mill MM 500 Nano
The mixer mill MM 500 is a compact, versatile bench-top unit which has been developed specially f...

Mixer Mill MM 400
The Mixer Mill MM 400 is a true multipurpose mill designed for dry, wet and cryogenic grinding of...

Benchtop Laboratory Homogenizer | M-110P Microfluidizer®
Microfluidizer® M110P Laboratory Homogenizer The Microfluidizer® M110P Processor is a premium, l...

Oils 7400 Dual Matrix Autosampler
One Autosampler for both oils and coolants Oils testing laboratories are adding more sample type...

AR 403 All-Purpose Equipment Drive Unit
The ERWEKA All-Purpose system is based on a powerful drive unit available in two versions. The va...

Retsch AS 200 Jet Pharma
For sieving specialists: With the AS 200 jet pro and the GMP-conform AS 200 jet pharma, laborator...

CAMSIZER ONLINE XL
The CAMSIZER ONLINE XL is a large particle size and shape analyzer that can measure particles ran...

Vibratory Disc Mill RS 300
The Vibratory Disc Mill RS 300 XL is suitable for the extremely quick, loss-free and reproducible...

High Shear Fluid Processor- Production Scale Biopharma fully cGMP-compliant
Key Features and Specifications: - Operating pressures up to 690 bar (10,000 psi), 1379 bar (20,0...

Planetary Stirrer PRS
The Planetary stirring unit for mixing of creams, ointments, pastes, moist powders and liquids. ...

HG-600 Geno/Grinder™ (SPEX #2010) High-Throughput Homogenizer
The HG-600 Geno/Grinder® 2010 high-throughput homogenizer and cell lyser is designed for rapid ce...

ultraWAVE Single Reaction Chamber (SRC) Microwave Digestion System
The remarkable new UltraWAVE features Milestone’s unique SRC technology in a fully automated benc...

traceCLEAN Acid steam cleaning system
Cleaning various accessories used in trace analysis work is a critically important laboratory rou...

ultraCLAVE Microwave Digestion System
The UltraCLAVE shares Single Reaction Chamber (SRC) technology with the UltraWAVE, offering all t...

Vibratory Disc Mill RS 200
The Vibratory Disc Mill RS 200 is suitable for the extremely quick, loss-free and reproducible gr...

Fluid Bed Dryer TG 200
To grind moist or even wet sample materials is not possible without bothersome side effects, espe...

Sample Divider PT 100
A faultless and comparable analysis is closely linked to accurate sample handling. Only a sample ...

Sample Splitters RT 6.5 - RT 75
A faultless and comparable analysis is closely linked to an accurate sample handling. Only a samp...

TF Tube Furnace Range
Carbolite Gero’s versatile new TF tube furnace range incorporates high-quality heating elements a...

Rotor Beater Mill SR 300
The Rotor Beater Mill SR 300 is suitable for coarse and fine size reduction, either in batches or...

Rotating Tube Divider PT 200
A faultless and comparable analysis is closely linked to accurate sample handling. Only a sample ...

Planetary Ball Mill PM 100
Planetary Ball Mills are used wherever the highest degree of fineness is required. Apart from the...

Planetary Ball Mill PM 400
Planetary Ball Mills are used wherever the highest degree of fineness is required. Apart from the...

Rapid Cooling Ovens
Rapid cooling ovens are frequently used for annealing thermo-luminescent dosimeters (TLD) that ha...

Planetary Ball Mill PM 200
Planetary Ball Mills are used wherever the highest degree of fineness is required. Apart from the...

PYRO Microwave Ashing System
Introducing the Milestone PYRO The PYRO is built to offer superior working conditions and a more...

Laboratory Chamber Furnaces High Temperature HTF Series
The HTF high temperature chamber furnace range comprises 1700 °C and 1800 °C models. The two sma...

Laboratory Ovens Standard AX Series
The Apex AX Series laboratory ovens offer an upper end temperature range of 250 °C in a benchtop ...

High Energy Ball Mill Emax
The Emax is an entirely new type of ball mill for high energy milling. The unique combination of ...

High Temperature Laboratory Ovens LHT Series
The LHT laboratory high temperature ovens comprise 3 sizes of bench mounted ovens, each available...

Mixer Mill MM 500 Control
The MM 500 control is a high energy laboratory ball mill that can be used for dry, wet and cryoge...

MultiVap 54 Automated Concentration System
The MultiVap 54, also known as "The Game Changer", is the latest multi-channel and multifunctiona...

Mortar Grinder RM 200
The Mortar Grinder RM 200 can mix and homogenize powders, suspension and pastes even with high vi...

Disc Mill DM 200
Thanks to its robust design, the Disc Mill DM 200 can be used under rough conditions in laborator...

TBH 425 Tablet Hardness Tester
The ERWEKA TBH 425 is a tablet hardness and combination tester for half-automatic measuring of up...

HC-8000 Pneumatic Laboratory Homogenizer
The HC series of air-driven homogenizers are lightweigh and easy-to-use and are suitable for batc...

Fan Convection Ovens Laboratory
Fan convection provides greater temperature uniformity and faster recovery rates than natural con...

VDT/S Vacuum Leak Tester
The VDT/S is a vacuum leak tester for blisters and other packaging forms. The maximum vacuum (abs...

TBH 325 Tablet Hardness Tester
Easy calibration Simple setup Calibration of the hardness test station is completely menu drive...

DEENA 3 Metals Digestion System
TheDEENA 3is manufactured by SEAL Analytical (Thomas Cain brand in Asia). SEAL Analytical manufac...

TBH 125 Tablet Hardness Tester
The TBH 125 is the ERWEKA basic device for testing tablet hardness (dual-mode: “constant speed” a...

XP-400 X-Press (SPEX #3636)
The XP-400 X-Press (SPEX #3636) 35-ton (31.8 metric ton) hydraulic laboratory pellet press that a...

EP-1 Tablet Press
EP-1 Overview The EP-1 is the ideal desktop tablet press for R&D purposes. The single punch ecc...

ST 35 Suppository Disintegration Testing
The ERWEKA suppository disintegration tester ST 35 comes with three turning test stations, each l...

SMG Manual Powder & Granule Density Tester
The manual ERWEKA SMG 697 is the unit for the reproducible determination of apparent bulk density...

Hammer Mill HM 200
The rugged RETSCH Hammer Mill HM 200 accepts large feed sizes up to 100 mm which can be reduced t...

Small and Pilot Scale Processor | M815 Microfluidizer®
The M815 is a pilot scale high shear fluid processor which achieves continuous operating pressure...

flexiWAVE Microwave Synthesis Platform
It is a common misconception that a laboratory microwave is just a microwave and that there are v...

Cutting Mill SM 100
Cutting mills are suitable for the grinding of soft, medium-hard, elastic, fibrous, and heterogen...

CG-400 Freezer/Mill (SPEX #6875)
The CG-400 Freezer/Mill (SPEX #6875) is a large cryogenic mill that accommodates sample sizes ran...

CG-900 Cryo-Blade™ Cryogenic Grinder
Cryo-Blade Cryogenic Grinder A cryogrinder is a laboratory mill used for grinding or homogenizin...

CG-500 Freezer/Mill (SPEX #6875D)
The CG-500 Freezer/Mill (SPEX #6875D) is a large high throughput cryogenic mill that accommodates...

Vibratory Sieve Shaker AS 450 Control
The analytical sieve shaker AS 450 control is used in research & development, quality control of ...

SimPrep Simple Automated Prep System
The SimPrep automated sample dilution system does more than just mixing and dispensing; it can au...

BD50 Block Digestion System
The SEAL Analytical Digestion Block is your key to successful kjeldahl digestions. The BD50 Diges...

Vibratory Sieve Shaker AS 450 Basic
The AS 450 basic, is a budget-priced alternative to the AS 450 control sieve shaker. The new siev...

Vibratory Sieve Shaker AS 200 Basic
The analytical sieve shakers of the series AS 200 are used in research & development, quality con...

Vibratory Sieve Shaker AS 200 Digit cA
The analytical sieve shakers of the series AS 200 are used in research & development, quality con...

Vibratory Sieve Shaker AS 300 Control
The analytical sieve shaker AS 300 control is used in research & development, quality control of ...

Tap Sieve Shaker AS 200 TAP
The analytical sieve shaker AS 200 tap is used in research & development, quality control of raw ...

Ultrasonic Baths UR
RETSCH's product range includes three sizes of ultrasonic baths for cleaning test sieves and grin...

MultiCheck 6 Fully Automated Tablet Hardness Testing Solution
The next step New technologies for a quiet all-rounder. The MultiCheck 6 offers ease of operati...

Pneumatic High Shear Fluid Microfluidizer® | M110Y
The M-110Y Microfluidizer processor is a lab machine that provides the highest shear rates of any...

ETHOS™ X Microwave Extraction System
The ETHOS X represents the state of the art in closed vessel microwave-assisted extraction. Unmat...

Hydraulic Pilot Scale Homogenizer/Processor | M-110EH
The M-110EH-30 is a mobile high shear fluid processor which achieves continuous operating pressur...

Sieve Shaker AS 200 Control
The analytical sieve shakers of the series AS 200 are used in research & development, quality con...

High Shear Laboratory Homogenizer | LM10 Microfluidizer®
LM 10 - Digitally Controlled High Shear Fluid Processor lab unit- Ideal for small sample volume m...

TGA Thermostep Thermogravimetric Analyzer
Thermogravimetry is a standard method to analyze organic, inorganic and synthetic materials. Ther...

ELEMENTRAC OH-p 2 Oxygen / Hydrogen Analyzer
The ELEMENTRAC OH-p determines oxygen and hydrogen in inorganic samplesby inert gas fusion in an ...

Planetary Ball Mill PM 300
Introducing the new Retsch Planetary Ball Mill PM 300! A robust and ergonomic benchtop model wit...

ELEMENTRAC ON-p 2 Oxygen / Nitrogen Analyzer
The ELEMENTRAC ON-pguarantees precise and fast sample analysis. The analyzer covers a wide range ...

ELEMENTRAC ONH-p 2 Oxygen / Nitrogen / Hydrogen Analyzer
The ELEMENTRAC ONH-p determines oxygen, nitrogen, and hydrogen in inorganic samplesby inert gas f...

Knife Mill GRINDOMIX GM 200
The new knife mill GRINDOMIX GM 200 is the ideal instrument for grinding and homogenizing foods a...

Knife Mill GRINDOMIX GM 300
The new knife mill GRINDOMIX GM 300 is the ideal instrument for the grinding and homogenization o...

Water Chillers
One of the most successful LabTech lines is now enhanced with a new lightweight portable chiller....

BELSORP MINI X BET Specific Surface Area & Pore Size Analyzer
The BELSORP MINI X measures the specific surface area/pore size distribution by volumetric gas ad...

BELSORP MR1 BET Surface Area Analyzer
The BELSORP MR1 is designed for rapid and convenient BET single-point measurement. Highly effici...

BELSORP MAX G Surface Area and Pore Size Distribution Analyzer
Quick, Easy Characterization of Powder Materials with the Highest Accuracy The BELSORP MAX G is ...

BELSORP HP High Pressure Gas Adsorption Instrument
The BELSORP HP analyzer is used for high-pressure adsorption measurement. The importance of high-...

PM 30 Suppository Penetration Tester
PM 30 Testing of softening time of suppositories The ERWEKA PM 30 measures the softening time o...

EasyCheck Fully Automated Hardness Tester
Fully automatic hardness testing EasyCheck is the new entry-level tablet combination tester that...

GT Series Flowability Tester of Powders and Granules
Three Devices, all options - Our Granulate flow tester series The devices of the ERWEKA granulat...

DMA 80 evo Direct Mercury Analyzer
The Milestone DMA-80 has been at the forefront of direct total mercury analysis for almost two de...

Pellet Press PP 40
Solid, high-quality pellets are an important precondition for reliable and meaningful XRF analysi...

BM-200 Mixer/Mill®, Mini (SPEX #5120)
The The BM-200 Mixer/Mill, Mini (SPEX #5120) is ideal for pulverizing the toughest rocks, minera...

CG-200 Freezer/Mill (SPEX #6775)
The CG-200 Freezer/Mill (SPEX #6775) is our small cryogenic mill that accommodates sample sizes r...

duoPUR and subPUR Acid Purification System
Think Pure High-purity acids are critical to controlling the analytical blank, the "Achilles hee...

SYNC Particle Size and Shape Analyzer
Introducing the Sync by Microtrac, the first synchronous size and shape particle analyzer that in...
AIM™ Quick Change Mould System
The AIM™Quick Change Mould System is used worldwide in the Polymer industry. This Flexible mould ...

Rotary Evaporators
The LabTech evaporation solutions introduce a new concept in routine distillation/evaporation pro...

Atomx XYZ VOC Sample Prep System
The Atomx XYZ is the second generation combined soil/water autosampler and purge and trap concent...

BELPYCNO Gas Pycnometer
The BELPYCNO is an analyzer for quick and reliable measurement of the true density of solid mater...
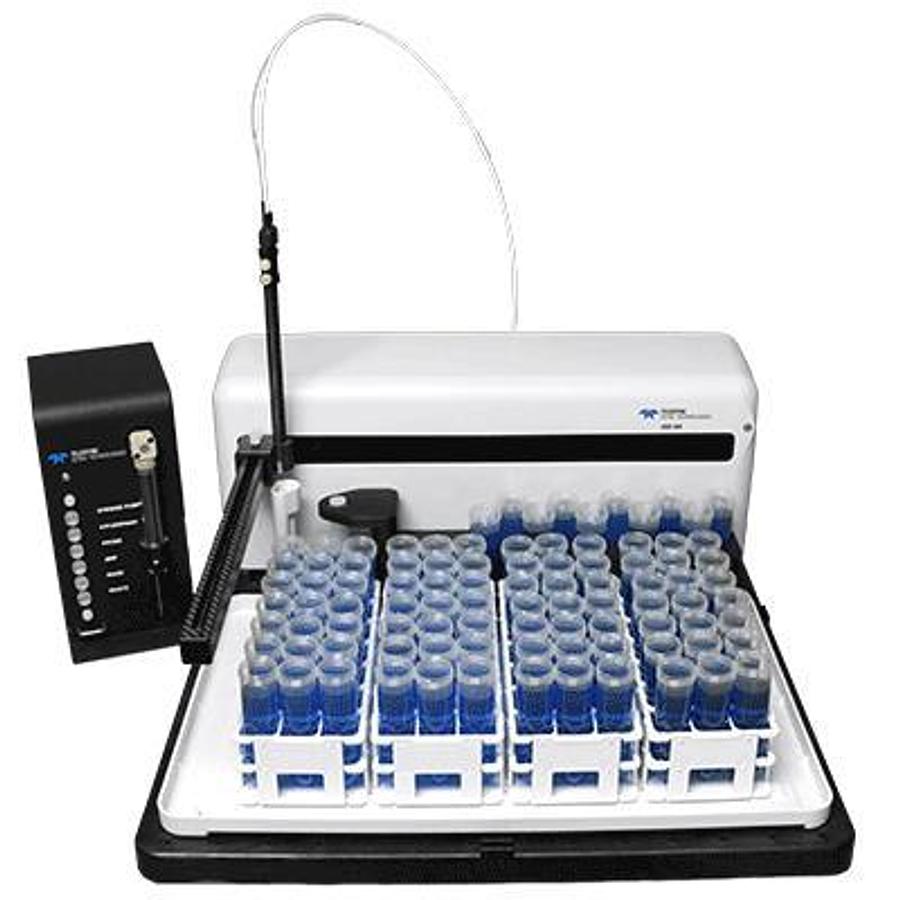
SDX High Performance Liquid Dilution System
An innovative spin on proven technology The SDXHPLDis a simple, straight-forward automated dilu...

Nanotrac Wave II Nanoparticle Size Analyzer
Featuring an enhancement to traditional DLS, Microtrac MRB’s Nanotrac Wave II employs Reference B...

BELMETAL 3 Metal Dispersion Rate Analyzer
METAL DISPERSION ANALYSIS Metal dispersion rate is one of the important factors to determine the...

BELSORP MAX X Series High-Precision Gas and Vapor Adsorption Analyzer
A lot of Science in a little space: BELSORP MAX X. High-End Adsorption. Lowest Footprint. Unrival...
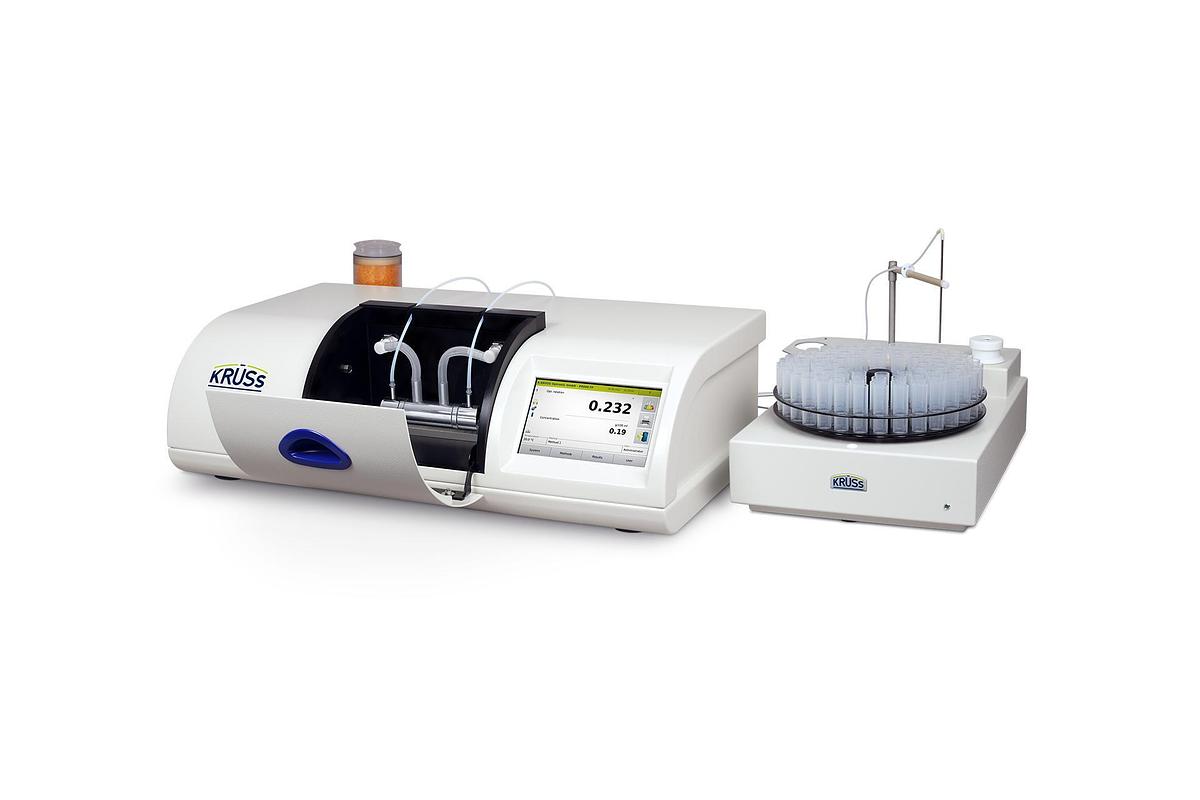
P8000 & P8100 Series Polarimeters
These rugged, high-end polarimeter models P8000-P and P8100-P, which otherwise have the same rang...

Melting Point Meters
The M5000 provides fast, easy and automatic examinationof powdery substances with a melting point...

STABINO ZETA Zeta Potential Analyzer
For the accurate determination of the zeta potential and colloidal stability, the STABINO ZETA is...

TURBISCAN LAB Stability Analyzer
The world reference stability analyzer TURBISCAN LAB enables fast and sensitive identification of...

BELPYCNO L Gas Pycnometer
BELPYCNO L is a fully automated gas pycnometer for the determination of volume and skeleton densi...

Acoustic and Electroacoustic Spectrometer DT 1202
DT are experts in instruments designed for performing particle size and zeta potential for charac...

Ultra-High Shear Manufacturing Processing | M700 Microfluidizer®
Ultra-High Shear Manufacturing Processing Once the suitable pilot batches are achieved, scale up...

Liquid Nitrogen Generators
"BREW" your own liquid nitrogen Make your own high-quality liquid nitrogen, anywhere, anytime. O...

CAMSIZER X2 Particle Size and Shape Analyzer
Quality control of fine powders can be substantially improved with the new CAMSIZER X2:Greater pr...

QuAAtro39 Segment Flow Analyzer
QuAAtro39 Continuous Segmented Flow Analyzer QuAAtro39 is the world's most automated, compact, C...

CN 802 Carbon Nitrogen Elemental Analyzer
The CN 802 is a robust and flexible combustion analyzer, that works in accordance with official r...

MiniLab Biochemical Oxygen Demand (BOD)
The MiniLab BOD Analyzer is the robotic analyzer boasting quick setup, precision robotics, true f...

CAMSIZER 3D Particle Size and Shape Analyzer
The CAMSIZER 3D particle analyzer combines all the advantages of dynamic image analysis (ISO 1332...

AQ700 Discrete Analyzer
Robotic Testing & Sample Handling Compact and powerful, the SEAL MiniLab AP (Auto Prep) and AR (...
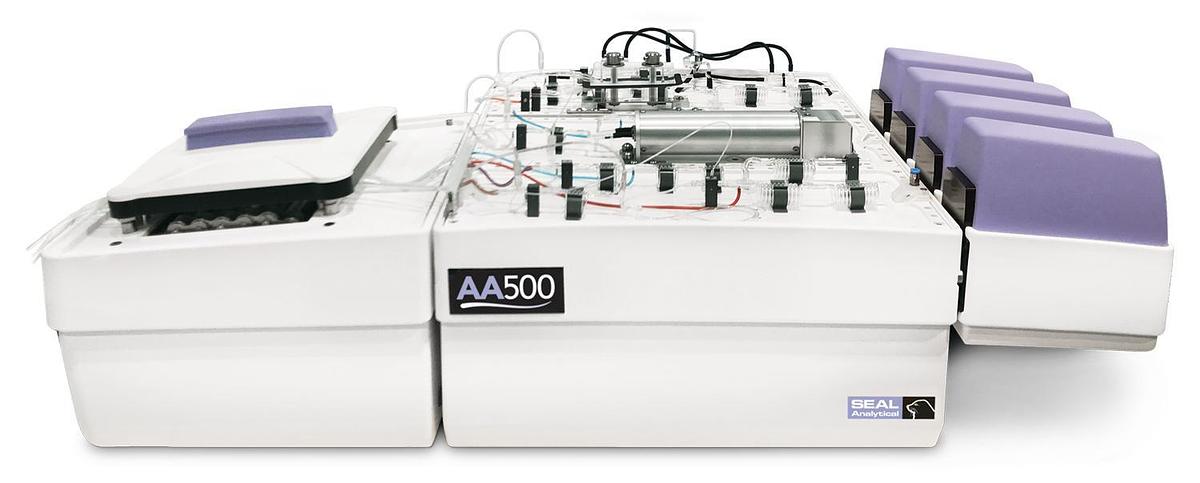
AA500 Segmented Flow Analyzer
The AA500 is the top-of-the-line AutoAnalyzer incorporating the latest innovations to deliver you...

AQ400 Discrete Analyzer
The AQ400 robotic sampling arm works in conjunction with a stepper motor-drivensyringe that is re...

AQ300 Discrete Analyzer
The AQ300 is a low cost flexible analyzer that uses the principle of discrete analysis where each...

AA100 Segment Flow Analyzer
The compact dual channel AA100 auto analyzer is the ideal chemistry analyzer for laboratories run...

Stereo Microscopes
A stereo microscope is sometimes mistaken for a simple binocular microscope.The binocular microsc...

EMA 502 Elemental Analyzer CHNS-O
Experience next-level elemental analysis with the VELP EMA 502, the ultimate solution for fast, a...

NDA 702 Dumas Nitrogen Analyzer
The NDA 702 Dumas elemental analyzer is the best solution for high throughput labs looking for a ...

AQUATek LVA Liquid Vial Autosampler
The AQUATek LVA is a full automation solution for routine analysis of waters by purge and trap co...

Nanotrac Flex Nanoparticle Size Analyzer
The Nanotrac Flex In-Situ Particle Size Analyzer Delivers Fast and Accurate Analysis Capability ...

PrepLinc Automated GPC/SPE and Evaporation System
Automated Sample Prep Systems with GPC/SPE & Evaporation Modules J2 Scientific, an international...
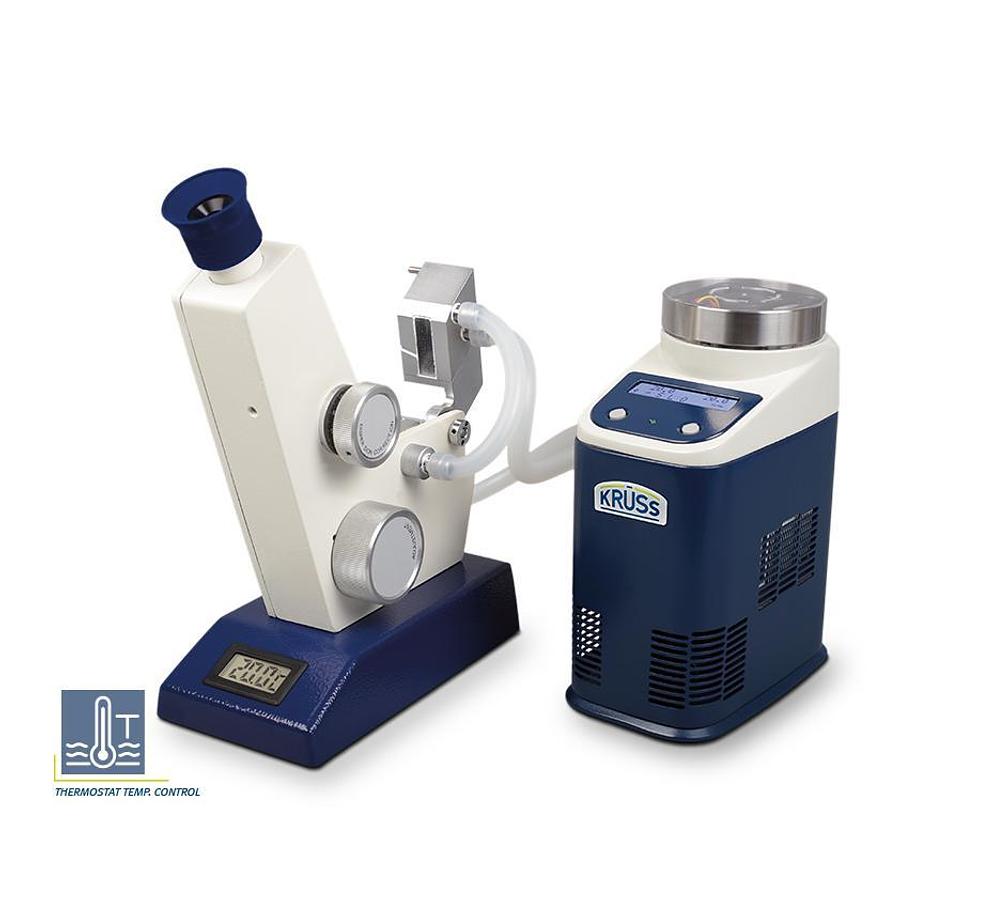
Digital Refractometer Series
Deliver highly accurate and reproducible measurement results, offer the option to work semi and f...

ASX‑280 Compact Autosampler
More features and improved performance define the next generation of autosampler from Teledyne CE...

DS7000 Series Digital Density Meter
Introducing A. KRÜSS Optronic's DS7000 Series of Digital Laboratory Density Meters for fast, accu...

PT80 Circulation Thermostat
For all basic tasks of temperature control in the laboratory The PT80 circulation thermostat is ...

CS-580A Carbon / Sulfur Analyzer
ELTRA‘s CS-580A is the ideal analyzer for the simultaneousdetermination of carbon and sulfur in o...

MP350 Microlyser™ Processor
Cells are the basic building blocks of all living things. From bacterial cells like E. Coli, to y...

ASX‑560 Autosampler
See why users call Teledyne CETAC Autosamplers the best on the market. Put the most reliable, lon...

APS-7650G Gravimetric Sample Prep Station
Automate ASTM D5185 and ASTM D4951 with the APS-7650G Gravimetric Sample Prep Station - Integrate...

LV1 - Low Volume Microfluidizer® Homogenizer
Responding to customer demand, Microfluidics has launched the LV1 low volume benchtop machine, wh...

APS-7450V Volumetric Sample Prep Station
The APS-7450V is made to keep pace with the increased speed of wear metals labs, where analysis t...

High Shear Fluid Processor for the Lab | LM20 Microfluidizer®
LM 20 - Digitally Controlled High Shear Fluid Processor lab homogenizer unit- Ideal for small sam...

FitoClima 600 & 1200 Plant Growth Chambers
PLANT GROWTH RESEARCH ‘REACH-IN’ENVIRONMENTAL CHAMBERS The FitoClima Bio chambers provide the co...

Cutting Mill SM 300
Cutting mills are suitable for the grinding of soft, medium-hard, tough, elastic, fibrous, and he...

Cutting Mill SM 200
Cutting mills are suitable for the grinding of soft, medium-hard, elastic, fibrous, and heterogen...

ZT 730 Series for Automated Disintegration Testing
The ERWEKA ZT 730 Series with cable-free AirBasket automatically determines the disintegration ti...

ZT 320 Series Disintegration Tester
On the ZT 320 disintegration testers series, the test stations (one to four) are driven individua...

ZT 120 Series Light Disintegration Tester
The ERWEKA ZT 120 light series is the perfect entry-level disintegration tester with one or two m...

YB-5 / VB-3 – Y/V Mixer
Mixer VB-3 with V-shaped mixing vessel out of stainless steel for gentle mixture of solid well fl...

Double Roll Crusher TG 2000
Double Roll Crusher with two driven rollers running in opposing directions from the inside to the...

TAR II Friability Tester
The TAR II is the next step in intelligent, upgradeable friability & abrasion testing according t...

High Speed Mixer SW 1/S
The Laboratory High Speed Mixer operates with the plough-share principle for mixing of powders, g...

SSP Melting Point Tester for Suppositories
SSP Melting point testing The ERWEKA SSP measures the melting point of suppository samples. It...

SBT 2 Suppository Hardness Tester
The suppository hardness tester (type SBT 2) consists of an electrically heated chamber with an i...

PRS – Planetary Mixer
The Planetary stirring unit for mixing of creams, ointments, pastes, moist powders and liquids. ...

Polishing Drum PT
Polishing Drum to polish coated tablets. The drum is covered with interchangeable felt parts. Th...

Pelletizer GTE
The pelletizer GTE (300 mm diameter, 9.5 litre) is attached to the AR 403 via the Universal Gear ...

MediPrep 820 & 1622 Automated Dissolution Media Prep
The MediPrep 820 series offers quick and easy preparation of up to 8 liters dissolution media in ...

DT Light Series Dissolution Testing
The ERWEKA DT light Series delivers the proven ERWEKA quality in a comprehensive package for a bu...

Laboratory Fume Hoods
Canadian Scientific’s ducted, filtered and work station extraction systems are designed to meet t...

Laboratory Countertops
Canadian Scientific Lab Systems is Canada’s premier distributor and installer of Durcon epoxy cou...

Laboratory Casework
We specialize in the design, supply and installation of laboratory furniture with an extensive ca...

Wet Granulator FGS II
Wet Granulator with oscillating rotor for the production of wet granules. The granules can be pr...

Export Manager Physical Tester Software
Simple and fast data export! Introducing the ERWEKA Export Manager: Simple and Fast Data Export!...

Drum Hoop Mixer RM
The drum hoop mixer features diagonal placement of the drum within the hoop and is available with...

DRT Chewing Gum Tester
he ERWEKA DRT is the perfect device for testing of in vitro releases of substances from chewing g...

Double Cone Mixer DKM
The Double Cone Mixer allows mixing of free flowing powders and granules. The arrangement of the...

DT 950 & DT 9510 Series Digital Dissolution Offline System
The Digital Offline System for DT 950 & 9510 Series is the next step to utilize the power of digi...

DT 9510 Series Digital Dissolution Tester
High volume dissolution testing with up to 14 test stations The ERWEKA DT 9510 Series is the big...

DFZ II Flow-Through-Cell
The new ERWEKA flow-through cell tester DFZ II can be used for various applications thanks to its...

Cube mixer (KB 15/20/S)
The Cube Mixer KB uses a tumbling motion to produce a homogenous blend within a short time. The m...

Coating Pans DKE/DKS
The ERWEKA coating pan is used for the development of coating tablets. The universal gear UG is r...

Conical Mill CM
The CM is designed for homogenization of dry products to a specified particle size, reduction of ...

BioDis RRT 10 Dissolution Tester
With the ERWEKA RRT 10, automatic dissolution testing of different extended and sustained release...

Ball Mill KM
The Ball Mill is used for grinding crystalline materials and for mixing dry, or under certain cir...

BELPREP VAC
BELPREP VAC is a vacuum degasser with flow degassing option. The instrument is optimized for the ...

XRD-Mill McCrone
The XRD-Mill McCrone was specially developed for the preparation of samples for subsequent X-ray ...

Rotating Sample Divider PT 300
A faultless and comparable analysis is closely linked to accurate sample handling. Only a sample ...

Jaw Crusher BB 400
The Jaw Crusher BB 400 XL is used for rapid, effective crushing and pre-crushing of medium-hard, ...

Jaw Crusher BB 600
The Jaw Crusher BB 600 XL is used for rapid, effective, crushing and pre-crushing of medium-hard,...

Jaw Crusher BB 500
The Jaw Crusher BB 500 XL is used forrapid, effective, crushing and pre-crushing of medium-hard, ...

Jaw Crusher BB 200
The Jaw Crusher BB 200 is used for the rapid, gentle crushing and pre-crushing of medium-hard, ha...

S3500 Particle Size Analyzer
The Microtrac S3500 is the first particle size analyzer that uses three precisely placed red lase...

Jaw Crusher BB 300
The Jaw Crusher BB 300 is used for the rapid, gentle crushing and pre-crushing of medium-hard, ha...

Jaw Crusher BB 50
The Jaw Crusher BB 50has been specially designed for sample preparation in the laboratory.It is u...

Jaw Crusher BB 250
The RETSCH BB 250 is a jaw crusher designed for primary size reduction of hard, brittle, and toug...

Drum Mill TM 300
The TM 300 Drum Mill is used for the preparation of granules and powders.The grinding process is ...

SLFA 60 XRF Sulfur-in-Oil Analyzer
The HORIBA SLFA 60 is a single sample X-ray fluorescence analyzer that delivers fast and accurate...

SLFA 6000/6800 Series XRF Sulfur-in-Oil Analyzers
The SLFA series are designed specifically to meet the recent demanding needs of measuring the new...

SM-200 Shatterbox, Enclosed (SPEX #8530)
The SM-200 Shatterbox, Enclosed (SPEX #8530) Ring & Puck mill with sound-proof enclosure that acc...
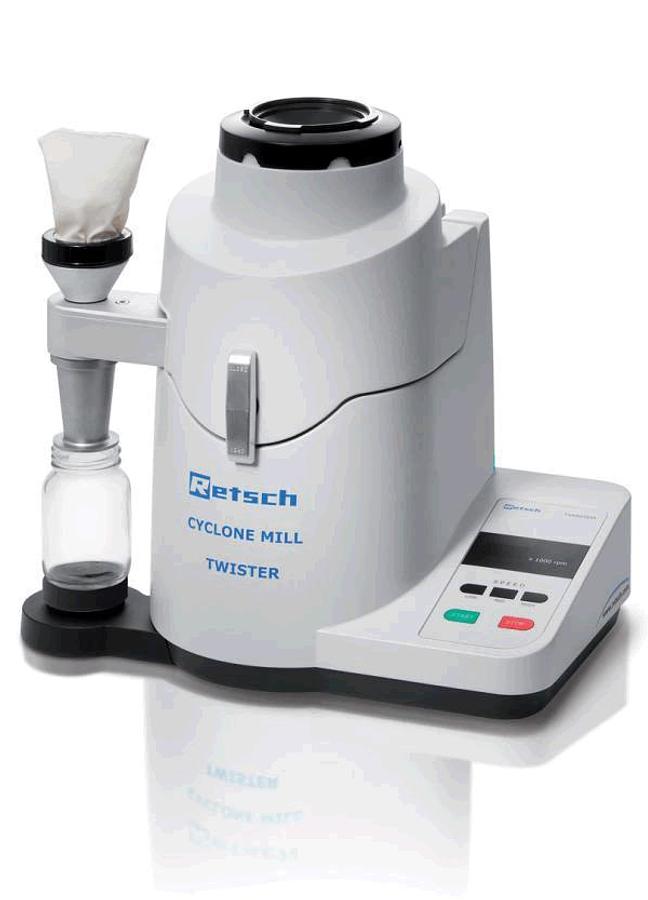
Cyclone Mill Twister
The Cyclone Mill TWISTER is specially designed for the processing of foods and feeds for subseque...

BM-450 Mixer Mill Dual (SPEX #8000D)
The BM-450 Mixer Mill Dual (SPEX #8000D) is a high-energy ball mill with dual clamps that accommo...

Clean Room Ovens
The CR Series of 250°C clean room ovens are available in 9 standard models in sizes ranging from ...

BM-400 Mixer/Mill® (SPEX #8000M) - High-Energy Ball Mill
The BM-400 Mixer/Mill (SPEX #8000M), is a high-energy ball mill that grinds up to 0.2 - 10 grams ...

Lotix Combustion TOC Analyzer
The Lotix TOC Combustion Analyzer is designed to accurately measure carbon content in aqueous mat...

SurfaceC‑800 Surface Carbon Analyzer
Determination of surface carbon is a special requirement for quality control in the metal produci...

BELPORE Series Mercury Porosimeter
The BELPORE mercury porosimeter series for low-pressure (LP), medium-pressure (MP) and high-press...

HG-400 (SPEX #1600) MiniG Tissue Homogenizer
The HG-400 (SPEX #1600) MiniGTissue Homogenizer is the ideal solution for labs that want a compac...

BELCAT II Catalyst Analyzer
All-in-One, Fully-Automatic and Multi-Purpose Analyzer The surface properties of solid catalyst ...

MESA-7220 XRF Sulfur/Chlorine-in-Oil-Analyzer
All of the compact bench top models utilize a new approach to EDXRF, Monochromatic Excitation-EDX...
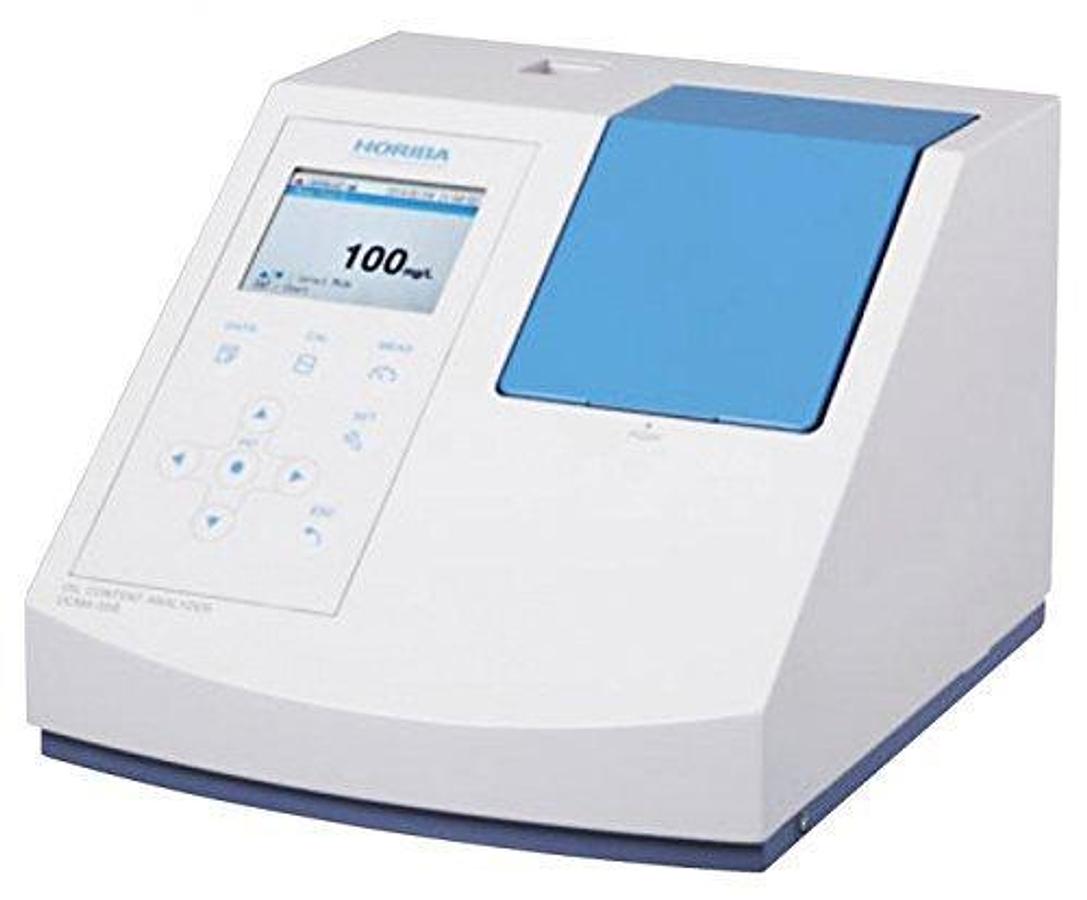
OCMA-550 Automatic Oil Content Analyzer
The OCMA-500 is a fully automated Oil content analyzer that quickly and accurately measures the c...

OCMA-500 Automatic Oil Content Analyzer
The OCMA-550 is a semi-automated Oil content analyzer that quickly and accurately measures oil de...

3621 Carver Manual Press
Full-size 12-ton (10.9 metric ton) Carver® hydraulic laboratory pellet press that accepts 13 mm, ...

3622 Carver Manual Press
Full-size 25-ton (22.7 metric ton) Carver® hydraulic laboratory pellet press that accepts 13 mm, ...

Calibration Standards for Petroleum Products
Analytical Services, Inc. specializes in custom formulation of multi-element standards in difficu...

easyFILL Automated Reagent Dosing Station
Sample preparation is a central part of elemental analysis and includes several manual and time c...

XELSIUS Reaction Solutions
XELSIUS is a compact and fast reactor with 10 completely individually temperedand stirred reactor...

OXITEST Oxidation Stability Reactor
Fats and oils oxidation stability tests for R&D and QC Labs The oxidation stability tests perfor...
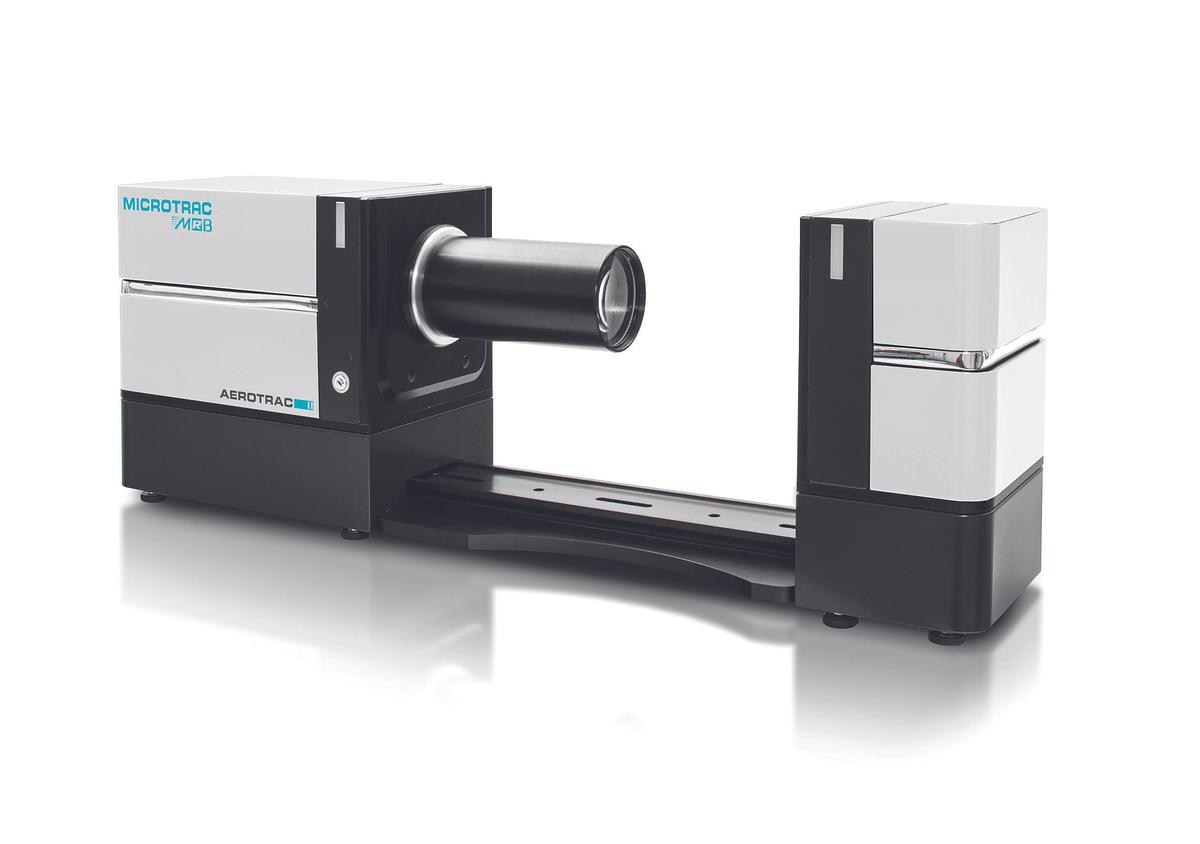
AEROTRAC II Spray particle & spray droplet size analyzer
The AEROTRAC II is an analyzer for particle size distributions & concentration ratio analysis (ca...

Air Jet Sieving Machine AS 200 Jet
The new Air Jet Sieve AS 200 jet is particularly suitable for sieve cuts of powdered materials wh...

BLUEWAVE Particle Size Analyzer
Microtrac MRB’s Bluewave provides accurate, reliable, and repeatable particle size analysis for a...

Cutting Mill SM 50
A single solution for everything Unleash the power of precision and efficiency with the SM 50, t...

Cross Beater Mill SK 300
The Cross Beater Mill SK 300 is suitable forcoarse and fine size reduction,either in batches or c...

Automatic Kjeldahl Digesters DKL Series
AUTOMATIC DIGESTION UNITS Sample preparation is a crucial part of the Kjeldahl analysis in order...

Semi-Automatic Kjeldahl Digesters DK Series
SEMI-AUTOMATIC DIGESTION UNITS DK digesters are traditional digesters, consisting of an aluminum...

Gas Analyzer Series
A.KRÜSS gas analyzers provide fast, reliable measurement results. They are used by many companies...

Flame Photometer Series
The A.KRÜSS flame photometers allow simple and cost-effective determination of alkali and alkalin...

UDK 169 with AutoKjel Autosampler
The UDK 169 is designed for high-throughput laboratories looking for precise and reproducible res...

UDK 159 Automatic Kjeldahl Analyzer
Fully automatic Kjeldahl Analyzer (distillation unit with integrated colorimetric titrator), for ...

SVM II Tapped Density Tester
The SVM II is our latest generation of tapped density tester. With unprecedented flexbility and i...

Torch Combustion TOC Analyzer
The Torchutilizes a patent pending Static Pressure Concentration (SPC) for the analysis of TOC us...

Lumin Purge and Trap Concentrator
The fundamentals of Purge and Trap are time proven. By bubbling a purge gas through your sample m...

ELEMENTRAC H-r
Determination of hydrogen using the heat extraction method is a special requirement for the chara...

CW-800M Carbon / Water Analyzer
ELTRA’s CW-800M analyzer is designed for fractional analysis of carbon and water in one single op...

Drum Mill TM 500
The Drum Mill TM 500 is a laboratory ball mill designed to grind large sample volumes up to 35 l....

ELEMENTRAC CS-I Carbon / Sulfur Analyzer
The elemental analyzer ELEMENTRAC CS-i measures the carbon and sulfur concentration in predominan...

Fusion UV/Persulfate TOC Analyzer
The Fusion Total Organic Carbon Analyzer utilizes powerful UV Persulfate oxidation allowing super...

CHS-580A Carbon / Hydrogen / Sulfur Analyzer
ELTRA‘s CHS-580A is the ideal analyzer for the simultaneous determination of carbon, hydrogen, an...

HG-200 GenoLyte® (SPEX #1200) Homogenizer and cell lyser
The GenoLyte is the ideal solution for labs that want a compact yet powerful tissue homogenizer a...

Cryomill Cryogenic Mill
The CryoMill is tailored for cryogenic grinding. The grinding jar is continually cooled with liqu...

PF Series Fan Convection Laboratory Oven
The PF460 has been specially designed for the drying and decarboxylation of cannabis for the phar...

BELCAT II Catalyst Analyzer
Mass Spectrometer for Qualitative and Quantitative Gas Analysis Mass spectrometry (MS) is an ana...

ETHOS™ XL - Superior Live Terpene Extraction
Superior Live Terpene Extraction Milestone's ETHOS XL microwave extraction solutions enable you t...




