News
ATS Scientific Inc. is excited to announce the appointment of Atri Maharaj as our new Vice President of Sales!
February 4, 2025

ATS Scientific is excited to announce the appointment of Atri Maharaj as our new Vice President of Sales & Marketing!
Atri brings a dynamic and diverse background in sales, leadership, coaching, IP law, and athletics. His strategic mindset, relationship-building expertise, and passion for growth make him a powerful addition to the ATS team.
A proven leader, Atri has built and led high-performing teams, consistently exceeded sales targets, and fostered cultures of collaboration and innovation. His experience in both corporate environments and competitive sports perfectly aligns with our guiding ethos: “Working together…Providing solutions!”
We are confident Atri will play a pivotal role in expanding our market presence, strengthening partnerships, and delivering even greater value to our customers.
We’d also like to recognize Gilles Groulx, who has served ATS for over 30 years in different capacities including Vice President of Sales and Marketing since 2014. Gilles will be retiring in the summer of 2025, and in the coming months, he will continue in his role to ensure a smooth and seamless transition of responsibilities.
Please join us in welcoming Atri and celebrating this exciting new chapter for ATS Scientific!
#Leadership #Sales #Growth #Innovation #Teamwork #WelcomeToTheTeam #VPofSales
Product highlight - LM10 Digitally Controlled High Shear Fluid Processor
December 17, 2024
Perfect for small sample material processing, the #LM10 combines effortless operation with exceptional results—no extensive training required!
✅ Step-by-step consistency for repeatable, reliable results across batches and operators.
✅ Easy maintenance with a compact, user-friendly design for simple cleaning and fewer replacement parts.
Engineered by Microfluidics experts, the LM10 saves you time and effort while delivering superior performance every time.

Products for the Food & Feed Industry
December 6, 2024

COLOUR READERS
Simple, Intuitive, Handheld Operation
The CR-400 Chroma Meter is a handheld, portable measurement instrumentdesigned to evaluate the colour of objects, particularly with smoother surfaceconditions or minimal colour variation. Also available, the CR-410 has a 50mmmeasuring area for non-homogeneous samples. The perfect solution for colourinspections of food applications within quality control, quality assurance, and R&Dfrom Konica Minolta.
Sample Processing
Grinding and Homogenizing Foods and Feeds
The GM 200 from Retsch Milling & Sieving is ideal for homogenizingfood and feed substances with a high water, oil or fat content as wellas being perfectly suited for grinding dry, soft and medium-hardproducts. The quick homogenization process without significanttemperature rise ensures preservation of volatile sample components.
Microwave Sample Prep
High Performance Microwave Digestion System
Designed primarily for closed vessel microwave acid digestion, Milestone's ETHOS UP offers productivity, safety, ease of use, connectivity, expertise andapplication flexibility. Featuring a comprehensive choice of accessories,offering a first-class solution for microwave solvent extraction, high-temperature fusion, vacuum evaporation and even organic and inorganicsynthesis or protein hydrolysis for food applications.
ATS Scientific Inc. Announces the Acquisition of Folio Instruments Inc.
December 5, 2024
BURLINGTON, ON, Nov. 2022 /CNW/ - ATS Scientific Inc. ("ATS Scientific" or "the Company"), one of Canada's largest independent providers of scientific equipment and service, is pleased to announce that it has acquired Folio Instruments Inc. ("Folio"), a Kitchener-ON based distributor of scientific equipment and service provider.
Co-founded by Gord Howes and Rhett Barriere over 30 years ago, Folio has been an integral part of the industry addressing the growing demand for laboratory equipment in Canada. Folio has a particularly strong presence in the petroleum, energy, environmental and heavy industrial industries, highly complementary to ATS' offerings. With a product portfolio consisting of market-leading manufacturers such as PAC LP, SEAL Analytical Inc., Hitachi High-Tech Analytical Science, Konica Minolta, and many others, the addition of Folio will keep ATS Scientific at the forefront of the scientific community.
"We are all excited to join ATS Scientific's growing national team. Right away, we knew there was a fit, as their like-minded focus on putting customers first and delivering superior solutions really resonated with us" commented Folio's Co-Founder and President, Gord Howes, who will continue to be an integral part of the Company. Rhett Barriere will continue on as a Technical Consultant and added "we look forward to helping accelerate the Company's growth and further strengthening its presence coast-to-coast".
ATS Scientific will continue to be led by CEO Aleks Sobot and VP, Sales Gilles Groulx with critical support from the company's equity investor Sage Capital Partners. On the acquisition, CEO Aleks Sobot commented, "The acquisition of Folio represents the ongoing execution of our growth strategy, significantly enhancing our presence in very favourable end markets. Folio has a sterling reputation known across Canada and deepens our resources significantly. I am thrilled to welcome the Folio staff to our growing team at ATS Scientific."
Integration has already begun with planned cross-training across all product lines, which will provide an enhanced level of support for current and future customers. Folio will continue to operate out of its Kitchener facility and will maintain its name, website, and e-commerce store.
About ATS Scientific
ATS Scientific Inc was founded in 1989 by Alex Heino as a small service organization and has evolved into a highly respected sales and service organization serving the Canadian laboratory marketplace with a comprehensive product and service portfolio. The company has been successful in creating a team of dedicated professional staff, who are by far its greatest asset, as well as long term relationships with valued customers and suppliers who have been fundamental to the company's success. Learn more at www.ats-scientific.com.
About Folio Instruments
Folio Instruments has been one of Canada's leading suppliers of scientific instruments since 1989. Originally a modest basement operation, Folio expanded to a national company with a physical presence in Kitchener, Ontario, Calgary, Alberta, and Montreal, Quebec. The company's focus has always remained steadfast in laboratory analytical instrumentation, developing a deep knowledge of our equipment and methods. The guiding principle of operation is "say what you do and do what you say". The Folio commitment is to always stand behind its instrumentation and its performance. Learn more at www.folioinstruments.com.
About Sage Capital Partners
Based in Toronto, Ontario, Sage Capital Partners is the first institutional fund focused on search fund investing in Canada. Sage supports both traditional searchers and non-traditional/self-funded entrepreneurs in their quest to acquire and grow companies in the lower mid-market. Sage has extensive experience in a wide variety of businesses and industries and continues to actively pursue control and minority investment opportunities. Learn more at www.sagecapfund.com.
SOURCE ATS Scientific
For further information: Aleksandar Sobot, asobot@ats-scientific.com
ATS Scientific Inc. is now the exclusive source for sales and service of ERWEKA products in Canada
January 5, 2024
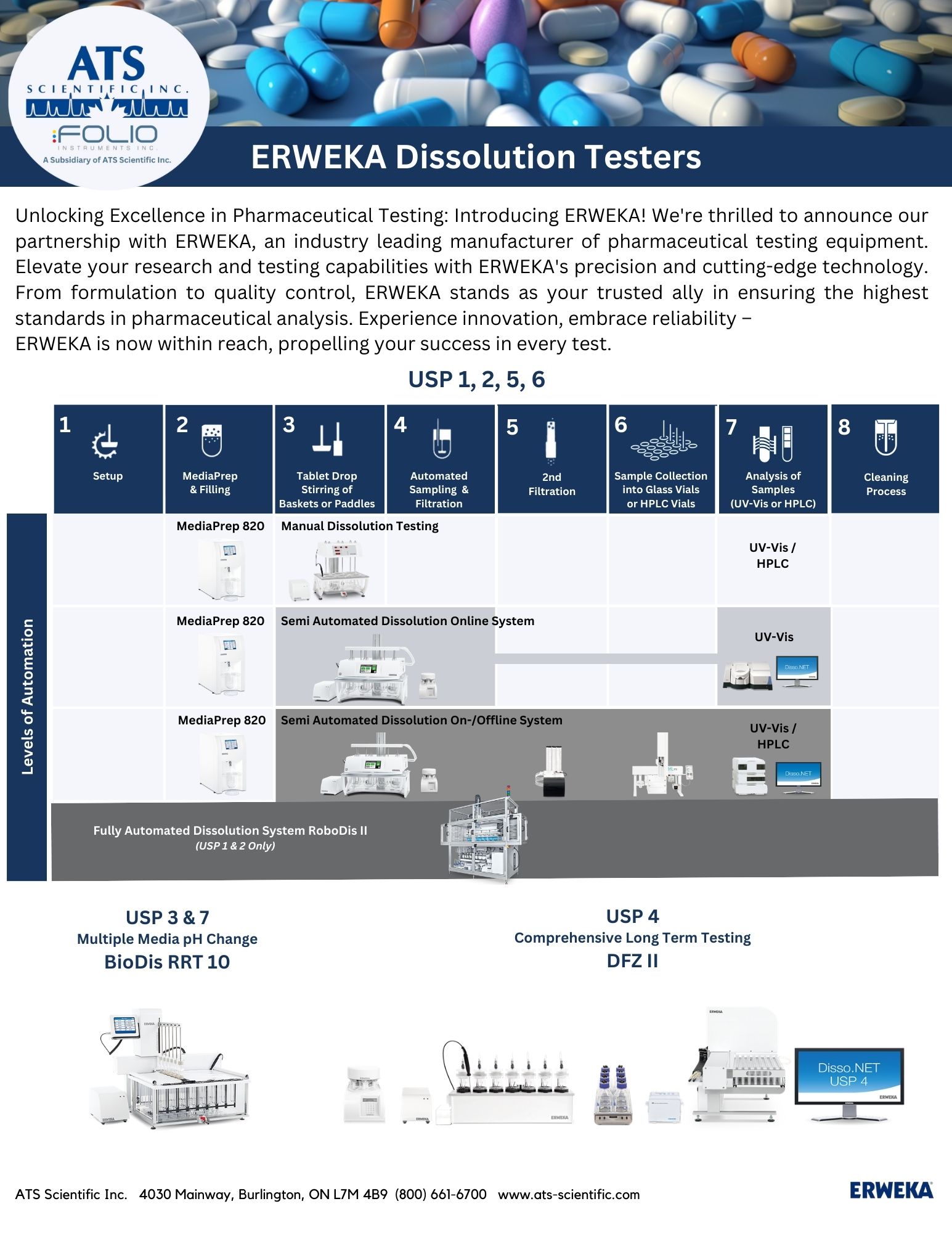
ATS Scientific Inc. (“ATS”) is pleased to announce that it is now the exclusive source for sales and service of ERWEKA products in Canada. ERWEKA, based in Germany and part of the Verder Scientific group of companies, is renowned for its development and manufacturing of premium pharmaceutical tablet testing equipment.
ERWEKA, founded in 1951, supplies dissolution and tablet testing equipment for pharmaceutical and life science companies, research and test laboratories, and universities all over the world.
Since 1989, ATS has been a leading provider of high-quality Analytical Instrumentation, Sample Preparation and Materials testing equipment sales and service across Canada. Our service team employs certified, factory-trained engineers for installations, repairs, preventative maintenance and extended warranties.
We are proud to be the sole source of sales and service for the entire ERWEKA product line across Canada as part of our goal of providing our customers with future-proof solutions for all of their laboratory needs.



Call Today 1-800-661-6700